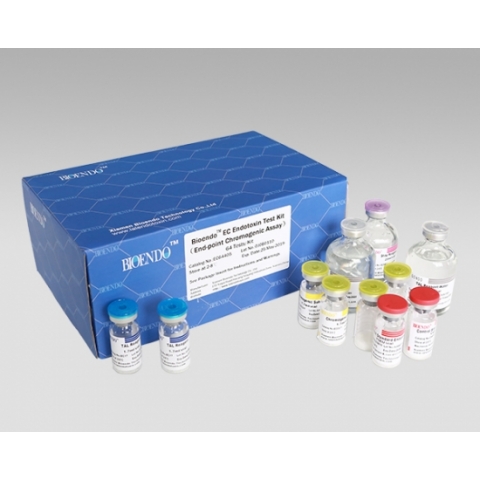
End-Point Chromogenic Endotoxin Assay
Bioendo is the first TAL reagent provider in China. The first TAL lysate provider licensed in China FDA.
The testing method conforms to USA/Europe/Japan Pharmacopoeia Bacterial Endotoxins Test .
1. Product information
End-point Chromogenic Endotoxin Assay is designed for the quantitative endotoxin assay without expensive endotoxin assay instruments. Using standard microplate reader with 405nm filter, could quantitative endotoxin level in 0.1-1ml range about 16 minutes.
The endpoint chromogenic assay kit contains TAL reagent , chromogenic substrate, Control Standard Endotoxin, stop reagent and TAL reagent water. This endotoxin detection method is strong resistant to interference, suitable for all kinds of biomedical products, such as protein, vaccine, plasmid, DNA, RNA endotoxin assay.
2. Product parameter
Sensitivity range:
0.01-0.1EU/ml: assay time about 46 minutes
0.1-1EU/ml: assay time about 16 minutes